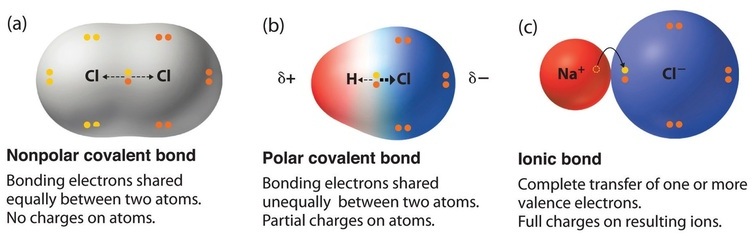
The hydrogen will sustain a slight positive charge and chlorine will sustain a slight negative one making the compound polar. Hydrochloric acid hcl is polar covalent because the electrons are more attracted to the chlorine and not the hydrogen.
 10 5 Molecular Shape And Polarity Chemistry Libretexts
10 5 Molecular Shape And Polarity Chemistry Libretexts
Cl on its own is a single atom and is not bonded to anything for there to be a difference in electronegativity.
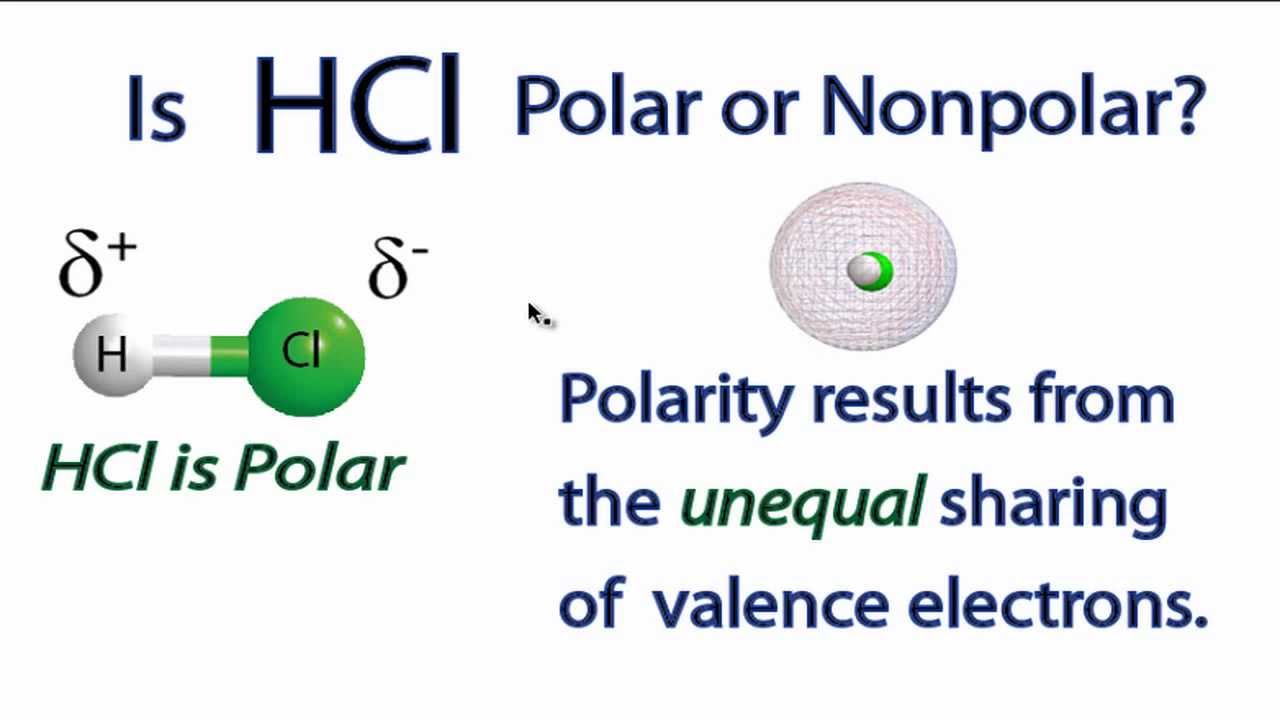
Hcl polar or nonpolar. Hcl hydrogen chloride is polar i ll tell you the polar or nonpolar list below. If you want to quickly find the word you want to search use ctrl f then type the word you want to search. Hcl or another name is hydrogen chloride which is very useful for industry or manufacturing.
Is hcl polar or nonpolar compound. Cl2 is nonpolar because there is no difference in electronegativity between atoms of the same element. Question is hcl hydrogen chloride polar or nonpolar.
Even without us knowing every day we use hcl or hydrogen chloride for household needs. Hcl is polar because there is a difference in electronegativity between hydrogen h and chlorine cl. The affinity to electrons is called electronegativity.
Hcl is a strong highly corrosive acid. Answer hcl hydrogen chloride is polar what is polar and non polar. Polar in chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.